Embark on an enlightening journey with our comprehensive Acid-Base Reaction Worksheet with Answers, meticulously crafted to enhance your understanding of these fundamental chemical interactions. Delve into the intricacies of acid-base reactions, exploring their types, strength, pH and pOH, and practical applications.
This worksheet is an invaluable resource for students, educators, and anyone seeking to master the concepts of acid-base chemistry. With a variety of engaging questions and detailed, step-by-step answers, it provides a thorough foundation for comprehending these essential reactions.
1. Introduction
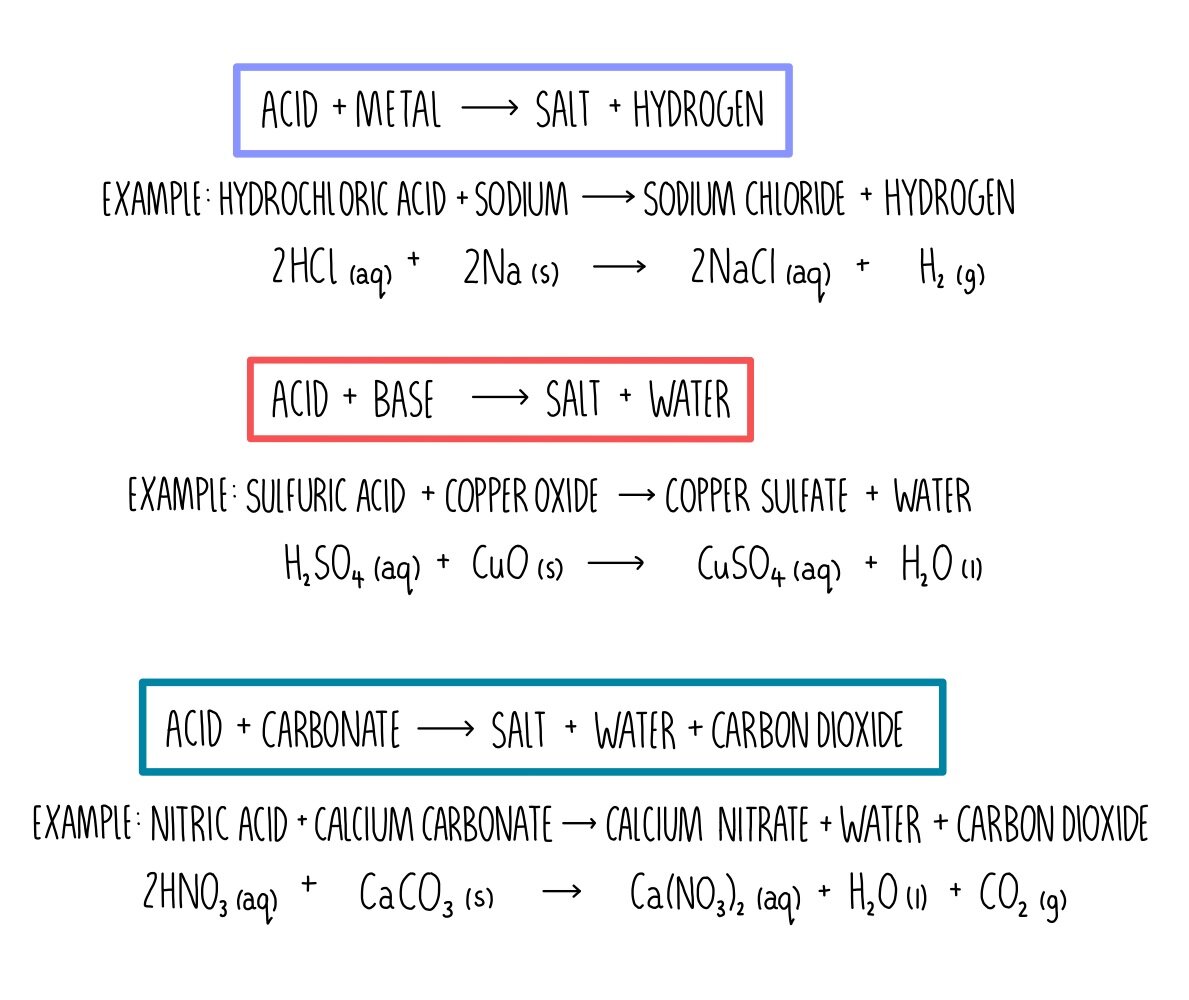
Acid-base reactions are chemical reactions that involve the transfer of protons (H+) between species. They are important in many areas of chemistry, including biochemistry, environmental chemistry, and industrial chemistry.
Understanding acid-base reactions is essential for a variety of reasons. First, they play a role in many natural processes, such as the regulation of pH in the human body and the weathering of rocks. Second, acid-base reactions are used in a wide range of industrial processes, such as the production of fertilizers, plastics, and pharmaceuticals.
Third, acid-base reactions can be used to analyze the composition of materials and to identify unknown substances.
2. Types of Acid-Base Reactions
There are three main types of acid-base reactions:
- Neutralization reactionsoccur when an acid and a base react in equal molar amounts to form a salt and water. For example, the reaction of hydrochloric acid (HCl) with sodium hydroxide (NaOH) produces sodium chloride (NaCl) and water (H2O):
HCl + NaOH → NaCl + H2O
- Precipitation reactionsoccur when two soluble ionic compounds react to form an insoluble solid precipitate. For example, the reaction of silver nitrate (AgNO3) with sodium chloride (NaCl) produces silver chloride (AgCl) precipitate and sodium nitrate (NaNO3) in solution:
AgNO3 + NaCl → AgCl (s) + NaNO3
- Gas evolution reactionsoccur when an acid reacts with a metal or a carbonate to produce a gas. For example, the reaction of hydrochloric acid (HCl) with zinc (Zn) produces hydrogen gas (H2) and zinc chloride (ZnCl2):
2 HCl + Zn → H2 (g) + ZnCl2
3. Acid-Base Strength
The strength of an acid or base is a measure of its ability to donate or accept protons. Strong acids donate protons easily, while weak acids donate protons with difficulty. Strong bases accept protons easily, while weak bases accept protons with difficulty.
The strength of an acid or base is determined by a number of factors, including:
- The electronegativity of the atom that donates or accepts the proton. The more electronegative the atom, the stronger the acid or base.
- The size of the atom that donates or accepts the proton. The larger the atom, the weaker the acid or base.
- The number of bonds that the atom that donates or accepts the proton can form. The more bonds the atom can form, the weaker the acid or base.
4. pH and pOH: Acid-base Reaction Worksheet With Answers
The pH of a solution is a measure of its acidity or alkalinity. The pH is defined as the negative logarithm of the hydrogen ion concentration ([H+]). The pOH of a solution is a measure of its basicity. The pOH is defined as the negative logarithm of the hydroxide ion concentration ([OH-]).
The pH and pOH of a solution are related by the following equation:
pH + pOH = 14
At 25°C, a neutral solution has a pH of 7 and a pOH of 7. A solution with a pH less than 7 is acidic, while a solution with a pH greater than 7 is basic.
5. Acid-Base Titrations
Acid-base titrations are used to determine the concentration of an unknown acid or base. In an acid-base titration, a known volume of a standard solution of an acid or base is added to a known volume of the unknown solution.
The endpoint of the titration is the point at which the moles of acid and base are equal. The concentration of the unknown solution can then be calculated using the following equation:
M1V1 = M2V2
where M1 is the concentration of the standard solution, V1 is the volume of the standard solution, M2 is the concentration of the unknown solution, and V2 is the volume of the unknown solution.
6. Applications of Acid-Base Reactions
Acid-base reactions are used in a wide variety of applications, including:
- The production of fertilizers. Acid-base reactions are used to produce fertilizers such as ammonium nitrate (NH4NO3) and potassium phosphate (K3PO4).
- The production of plastics. Acid-base reactions are used to produce plastics such as polyethylene (PE) and polyvinyl chloride (PVC).
- The production of pharmaceuticals. Acid-base reactions are used to produce pharmaceuticals such as aspirin and ibuprofen.
- The analysis of the composition of materials. Acid-base reactions are used to analyze the composition of materials such as food, water, and soil.
- The identification of unknown substances. Acid-base reactions are used to identify unknown substances by comparing their reactions to the reactions of known substances.
Commonly Asked Questions
What are the different types of acid-base reactions?
Acid-base reactions can be classified into three main types: neutralization, precipitation, and gas evolution reactions.
How do you calculate pH and pOH?
pH is calculated as the negative logarithm of the hydrogen ion concentration, while pOH is calculated as the negative logarithm of the hydroxide ion concentration.
What is the purpose of acid-base titrations?
Acid-base titrations are used to determine the concentration of an unknown acid or base by reacting it with a known concentration of a strong acid or base.
