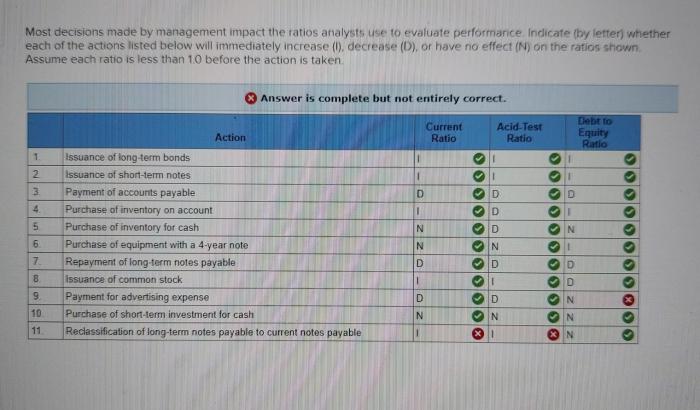
Indicate whether each item would increase or decrease contractility – Delving into the realm of muscle contractility, this exploration examines the intricate interplay of factors that govern the ability of muscles to contract and generate force. From the influx of calcium ions to the interactions between actin and myosin, we unravel the mechanisms that underpin muscle function, shedding light on the physiological processes that drive movement and maintain homeostasis.
As we delve deeper into the topic, we will explore how sarcomere length, ATP levels, and temperature exert their influence on contractility, providing a comprehensive understanding of the factors that shape muscle performance.
Effects of Calcium Ions on Contractility: Indicate Whether Each Item Would Increase Or Decrease Contractility
Calcium ions play a crucial role in muscle contraction. They bind to receptors on the surface of the sarcoplasmic reticulum, causing it to release calcium ions into the cytoplasm. This increase in calcium ion concentration triggers a series of events that ultimately lead to muscle contraction.
Mechanisms of Increased Contractility
- Increased calcium binding to troponin C:Calcium ions bind to troponin C, causing a conformational change that exposes the myosin-binding site on actin.
- Increased myosin-actin interaction:The exposed myosin-binding site allows myosin heads to bind to actin, forming cross-bridges.
- Power stroke:Myosin heads undergo a power stroke, pulling the actin filaments towards the center of the sarcomere, shortening the muscle fiber and generating force.
Impact of Sarcomere Length on Contractility
Sarcomere length is the distance between two adjacent Z-lines in a myofibril. It is an important determinant of muscle contractility.
Optimal Sarcomere Length
There is an optimal sarcomere length for maximum contractility. When the sarcomere is too short, the actin and myosin filaments overlap excessively, preventing the myosin heads from binding to actin. When the sarcomere is too long, the actin and myosin filaments overlap too little, again preventing cross-bridge formation.
Role of Actin and Myosin Interactions in Contractility
Actin and myosin are the two main proteins involved in muscle contraction. Actin filaments are thin and contain binding sites for myosin heads. Myosin filaments are thick and contain motor domains that generate force by interacting with actin.
Force Generation
The interaction between actin and myosin generates force through a series of steps:
- Myosin head binding:Myosin heads bind to the myosin-binding site on actin.
- Power stroke:The myosin head undergoes a power stroke, pulling the actin filament towards the center of the sarcomere.
- ADP release:ADP is released from the myosin head, causing it to detach from actin.
- ATP binding:ATP binds to the myosin head, causing it to reattach to actin and repeat the cycle.
Influence of ATP on Contractility
ATP is the energy source for muscle contraction. It is used to power the power stroke of the myosin head.
ATP Depletion and Contractility
When ATP levels are depleted, the power stroke cannot occur, and muscle contraction ceases. This can occur during intense exercise or in conditions such as hypoxia or ischemia.
Effects of Temperature on Contractility
Temperature has a significant effect on muscle contractility. As temperature increases, muscle contraction speed and force increase. This is because the rate of ATP hydrolysis and the power stroke are both temperature-dependent.
Temperature and Muscle Function, Indicate whether each item would increase or decrease contractility
In cold environments, muscles are less efficient and can become fatigued more easily. In warm environments, muscles can contract more quickly and forcefully, allowing for greater power output.
FAQ Summary
What is the role of calcium ions in muscle contraction?
Calcium ions play a crucial role in initiating muscle contraction by binding to receptors on the sarcoplasmic reticulum, triggering the release of calcium into the cytosol. This increase in calcium concentration allows for the formation of cross-bridges between actin and myosin filaments, enabling muscle contraction.
How does sarcomere length affect contractility?
Sarcomere length, the distance between adjacent Z-lines, influences the overlap between actin and myosin filaments. Optimal sarcomere length allows for maximum overlap and, consequently, greater contractile force. Deviations from this optimal length, either due to shortening or lengthening, result in decreased contractility.
What is the significance of actin-myosin interactions in contractility?
Actin and myosin are the primary proteins involved in muscle contraction. Actin filaments form the thin filaments, while myosin filaments form the thick filaments. The interaction between these filaments, facilitated by the motor protein myosin, generates the force necessary for muscle contraction.
How does ATP influence contractility?
ATP serves as the energy source for muscle contraction. It is hydrolyzed by myosin during the power stroke, providing the energy required for the movement of actin filaments and the generation of contractile force. Depletion of ATP levels impairs muscle function and reduces contractility.
What is the effect of temperature on contractility?
Temperature affects muscle contractility in a complex manner. Moderate increases in temperature can enhance contractility by increasing the rate of ATP hydrolysis and the kinetics of cross-bridge formation. However, extreme temperatures can denature proteins and disrupt muscle function, leading to decreased contractility.