Acids bases and the ph scale worksheet answer key – Acids, Bases, and the pH Scale Worksheet Answer Key: A Comprehensive Guide delves into the fundamental concepts of acids, bases, and the pH scale, providing a comprehensive understanding of their properties, applications, and safety considerations. This guide offers detailed answers to worksheet questions, exploring the significance of acids and bases in various fields and encouraging further exploration of this fascinating topic.
Acids and bases play a crucial role in our everyday lives, from the foods we eat to the products we use. Understanding their properties and behavior is essential for scientific research, technological advancements, and ensuring safety when working with these substances.
1. Acids, Bases, and the pH Scale
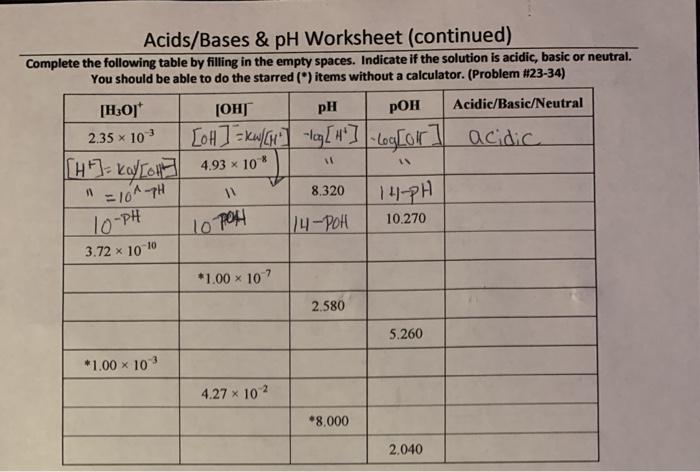
Acids are substances that release hydrogen ions (H+) when dissolved in water, while bases release hydroxide ions (OH-) when dissolved in water. Acids and bases have opposite properties and can neutralize each other. The pH scale is a measure of the acidity or alkalinity of a solution, with a pH of 7 being neutral, a pH below 7 being acidic, and a pH above 7 being alkaline.
Examples of Common Acids and Bases
- Acids: hydrochloric acid (HCl), sulfuric acid (H2SO4), nitric acid (HNO3)
- Bases: sodium hydroxide (NaOH), potassium hydroxide (KOH), ammonia (NH3)
2. Worksheet Answer Key
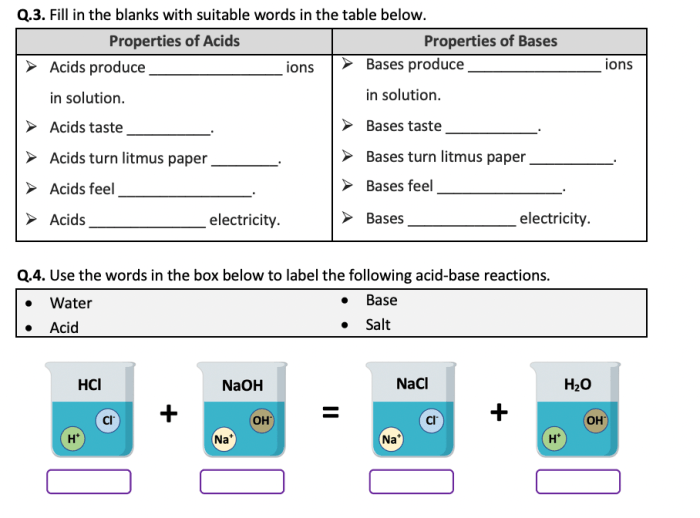
The worksheet covers the key concepts of acids, bases, and the pH scale, including their definitions, properties, and examples. It also includes questions on the pH scale and how to use it to determine the acidity or alkalinity of a solution.
Answers to Worksheet Questions, Acids bases and the ph scale worksheet answer key
- Question:What is the definition of an acid?
- Answer:An acid is a substance that releases hydrogen ions (H+) when dissolved in water.
- Question:What is the pH of a neutral solution?
- Answer:7
- Question:Which of the following is a strong acid? (a) HCl (b) CH3COOH (c) H2CO3
- Answer:(a) HCl
3. Applications of Acids and Bases
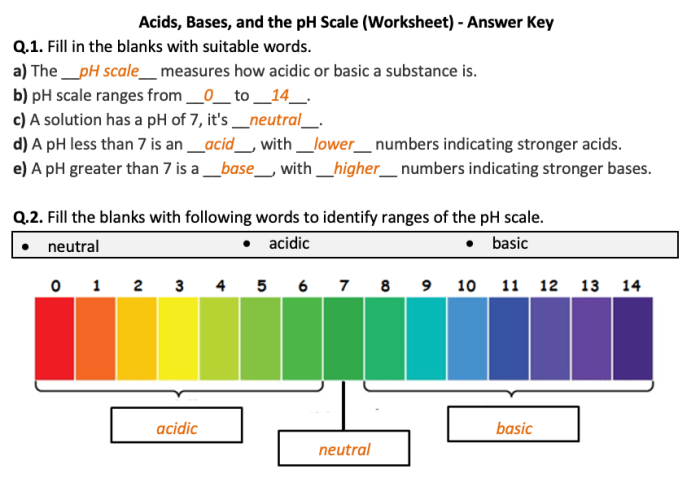
Acids and bases have a wide range of applications in various fields, including chemistry, industry, and medicine.
Chemistry
- Acids are used as catalysts in chemical reactions.
- Bases are used to neutralize acids and to produce salts.
Industry
- Acids are used in the production of fertilizers, dyes, and plastics.
- Bases are used in the production of paper, soap, and detergents.
Medicine
- Acids are used to treat indigestion and heartburn.
- Bases are used to neutralize stomach acid and to treat skin irritations.
4. Safety Considerations
Acids and bases can be hazardous if not handled properly. It is important to wear appropriate safety gear, such as gloves and eye protection, when working with acids and bases.
Potential Hazards
- Chemical burns
- Eye damage
- Respiratory irritation
Guidelines for Safe Handling, Storage, and Disposal
- Always wear appropriate safety gear.
- Store acids and bases in a cool, dry place.
- Dispose of acids and bases properly according to local regulations.
5. Further Exploration: Acids Bases And The Ph Scale Worksheet Answer Key
To learn more about acids, bases, and the pH scale, you can explore the following resources:
Quick FAQs
What is the pH scale?
The pH scale is a measure of the acidity or alkalinity of a solution, ranging from 0 to 14. A pH of 7 is neutral, while values below 7 indicate acidity and values above 7 indicate alkalinity.
What are the common examples of acids and bases?
Acids are substances that donate hydrogen ions (H+), such as hydrochloric acid (HCl) and sulfuric acid (H2SO4). Bases are substances that accept hydrogen ions, such as sodium hydroxide (NaOH) and potassium hydroxide (KOH).
Why is it important to understand acids and bases?
Acids and bases are essential for many chemical reactions and play a vital role in biological processes, industrial applications, and environmental chemistry. Understanding their properties and behavior is crucial for scientific research, technological advancements, and safety considerations.